Are you hoping to find 'how to write the formula for ionic compounds'? Here you can find questions and answers on this topic.
Table of contents
- How to write the formula for ionic compounds in 2021
- How to form ionic compounds
- How to do ionic compounds
- How to write a chemical formula
- Writing formulas for ionic compounds worksheet
- Writing formulas for ionic compounds answers
- In the chemical formula for an ionic compound, which item is written first
- Ionic compound formula examples
How to write the formula for ionic compounds in 2021
 This image shows how to write the formula for ionic compounds.
This image shows how to write the formula for ionic compounds.
How to form ionic compounds
 This image representes How to form ionic compounds.
This image representes How to form ionic compounds.
How to do ionic compounds
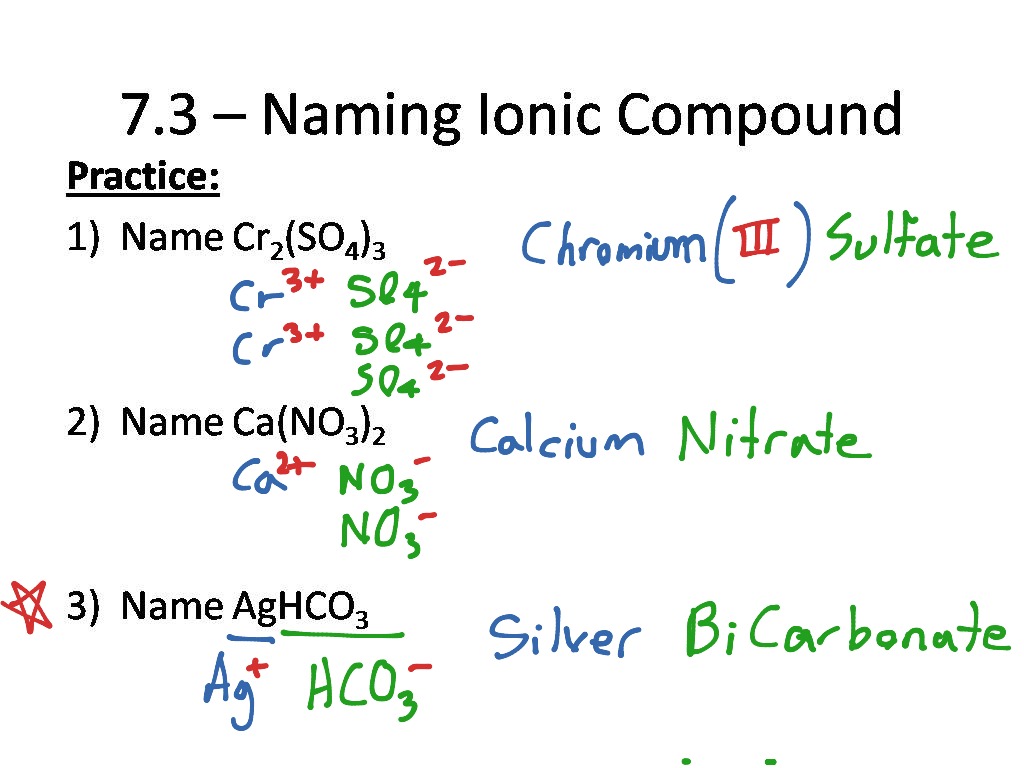 This image representes How to do ionic compounds.
This image representes How to do ionic compounds.
How to write a chemical formula
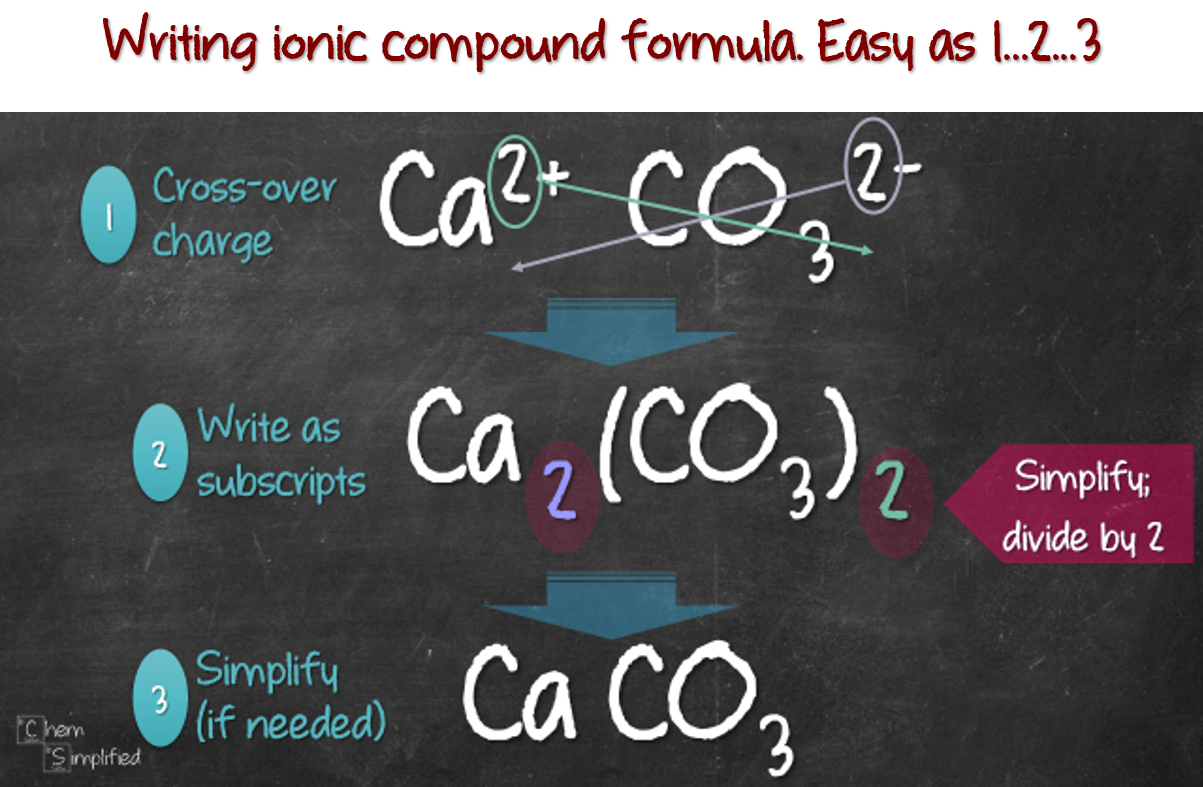 This image demonstrates How to write a chemical formula.
This image demonstrates How to write a chemical formula.
Writing formulas for ionic compounds worksheet
.PNG) This picture representes Writing formulas for ionic compounds worksheet.
This picture representes Writing formulas for ionic compounds worksheet.
Writing formulas for ionic compounds answers
 This image representes Writing formulas for ionic compounds answers.
This image representes Writing formulas for ionic compounds answers.
In the chemical formula for an ionic compound, which item is written first
 This picture shows In the chemical formula for an ionic compound, which item is written first.
This picture shows In the chemical formula for an ionic compound, which item is written first.
Ionic compound formula examples
.PNG) This image illustrates Ionic compound formula examples.
This image illustrates Ionic compound formula examples.
How to calculate the subscript of a polyatomic ion?
1. Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second. 2. Transpose only the number of the positive charge to become the subscript of the anion and the number only of the negative charge to become the subscript of the cation. 3. Reduce to the lowest ratio.
How to write formulae for simple ionic compounds?
As another example, if you knew that the charge on a sodium ion was +1, Na+, and the charge on an oxide ion was 2-, O2-, then it is easy to see that the formula for sodium oxide is Na2O. You need to have two sodium ions to balance the charges on the oxide ion.
Is the rule for ionic compounds the same for polyatomic ions?
The rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing monatomic (single-atom) ions: the positive and negative charges must balance.
How to write ionic charge based on name?
Write ionic charge based on the Roman Numeral in the name. For example, the Iron (II) ion would be Fe 2+. Polyatomic ions are made up of two or more elements (they will be non-metals).
Last Update: Oct 2021